Fish kills occur in natural and cultured populations. They can be due to disease, poor water quality or a toxic substance. Fish kills represent emergencies, and producers must be prepared for them in advance.
How can I be prepared for a fish kill involving disease or toxic substances?
Producers may make the following preparations to minimize fish losses:
- Determine the normal water quality of a pond or lake by checking water regularly with a water test kit.
- Be able to tell whether observed effects are from poor water quality, fish diseases or toxic substances.
- Know the telephone numbers and addresses of fish-disease and toxic-substance diagnostic laboratories.
- Know how to collect and preserve samples. Have approved sample containers and shipping cartons on hand.
- Know the proper chain-of-custody procedures for packaging and shipping samples, which are important in case of legal action.
- Determine the best method of transportation to have samples arrive within specified laboratory holding times.
Fish can behave erratically, get sick or die in response to toxic substances, poor water quality and infectious diseases. Although poor water quality and toxic substances can, by themselves, cause fish to get sick and die, infectious diseases involve a pathogen and some form of stress or adverse condition.
What is a toxic substance?
A toxic substance is any matter with harmful effects on living organisms. Such substances can be introduced into the aquatic environment by pesticide runoff, livestock feedlot discharge, oil from drilling or refining operations or acid water discharge from coal mining operations, to name a few. The observable effects can range from erratic fish behavior to massive fish kills.
Toxic substances are in either of two major categories: organics, such as most pesticides and herbicides, or inorganics, such as heavy metals. When a toxic substance is suspected, it is crucial that samples be handled properly to ensure the integrity of the sample and of the laboratory results.
Toxic substances may kill fish when water quality is good and no infectious disease is apparent. Producers must investigate a fish problem by going through a process of elimination that involves careful investigation of water quality, infectious disease and toxic substances (Figure 1). The first step is to look at water quality.
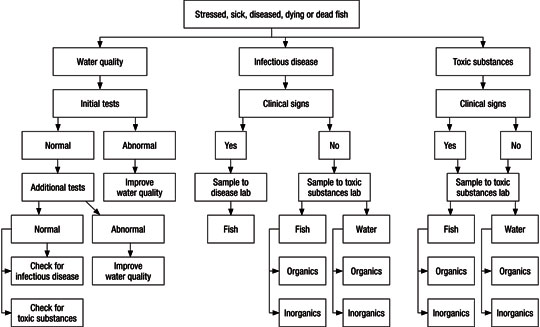
Figure 1
The process of investigative elimination to determine the cause of a fish health problem.
How can I rule out water quality problems?
Poor water quality can cause massive fish kills. It is often a major factor contributing to fish disease and parasite infection. Water quality does not remain constant. A pond's water quality may change drastically within a few hours.
Commercial fish producers should routinely monitor the water quality of their facilities with a water test kit. If fish are stressed or dying, immediately test the water for dissolved oxygen, carbon dioxide, conductivity, pH, temperature, ammonia nitrogen, nitrite nitrogen, alkalinity and hardness. Ideally, producers should know additional water quality parameters for their water, including color, biological oxygen demand (BOD), calcium, iron, manganese, nitrate nitrogen, total suspended solids, turbidity, sulfate, chloride and phosphate. Many of these tests can be performed by the producer with a complete water quality test kit.
For tests outside the scope of most test kits, contact your local MU Extension center for information on sampling, preservation and laboratories that perform this work.
What is an infectious fish disease?
Fish, like all animals, are subject to a variety of diseases. A host (fish), a pathogenic organism and a stressful environment combine to produce a fish disease. Pathogens rarely overwhelm a healthy population of fish, and they are capable of causing disease only when the host's resistance is lowered. Fish pathogens include parasites, bacteria and viruses. In addition, fish can suffer from environmental and nutritional diseases.
Fish seldom suffer from severe disease outbreaks under natural conditions. However, when fish are crowded and reared under unnatural conditions, the potential exists for a serious disease outbreak.
What are some causes of fish diseases?
Some predisposing factors leading to fish diseases may include stress from a rapid change in water temperature, pollution, unfavorable water chemistry (especially low oxygen), die-off of an algal bloom and a possible drop in pH after a heavy rain. Other adverse conditions that may trigger a disease outbreak include overstocking, poor handling procedures, overfeeding or underfeeding and poor food quality. Certain diseases also are more likely to occur as a result of seasonal changes of water temperature.
Intensively raised fish are most often stressed by these factors. Fish can handle the stress up to a point, but when they can no longer adjust, they may succumb to disease and eventually die.
How can disease be prevented?
Disease prevention is a primary goal in aquaculture. Ideally, the fish facility should be free of pathogens, but this is not always the case. If you purchase the fish, always obtain healthy fish from a reputable dealer. Stock the fish in an environment that has the proper water temperature, sufficient oxygen, low levels of ammonia and minimal organic loading. Overfeeding fish results in an accumulation of food that causes poor water quality.
Do not handle the fish too often or too roughly because this results in stress that may trigger diseases. You may incorporate preventive methods to keep stress to a minimum. For example, reduce stress from transportation and handling by adding 1,000 to 10,000 parts per million (ppm) salt and enough calcium chloride to raise the total water hardness to 50 ppm for soft water. Additional calcium chloride is not needed for harder water.
How can I recognize infectious diseases in fish?
Diseases occur despite the best preventive efforts. Detecting the diseases as soon as possible is vital. The best way to detect diseases at their onset is by watching the fish feed.
If you observe any of the following behavioral or physical signs, investigate immediately because a disease outbreak may be occurring:
Behavioral signs
- Failure to feed properly
- Flashing (turning on their sides)
- Rubbing on the bottom
- Lying listlessly in shallow water
- Gathering around the water inflow
- Reduced vitality
- Gasping at the surface
- Swimming erratically
Physical signs
- Blistered areas
- Swollen bellies
- Open sores (may be bloody)
- Popped-out eyes
- Bloody (hemorrhaged) areas on fins
- Discoloration or erosion of body parts
- Excessive mucus
- Growths on the body
Carefully monitor and record fish mortality rates daily. Records indicate the trend of the disease and provide clues as to the cause. Figure 2 illustrates the relationship between percent mortality and days that may help identify the cause of the problem.
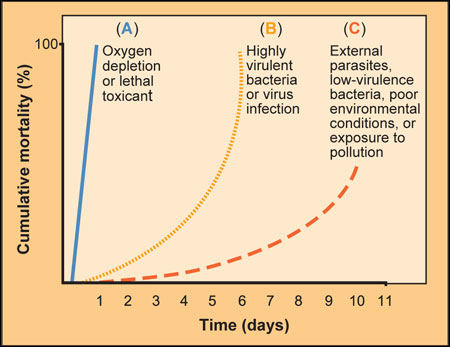
Figure 2
Measuring the relationship between percent mortality and time can help in diagnosing fish diseases
Line A represents die-off due to a severe environmental problem, such as low oxygen or a toxic chemical. Line B represents die-off due to an acute disease outbreak, such as bacteria. Line C represents die-off due to a chronic disease, such as external parasites. A diagnostic laboratory can use this information to help distinguish between various causes of fish mortality.
If infectious disease is present, how many fish samples should I collect?
Collect one sample consisting of three fish to six fish that exhibit some of the abnormal or erratic behavior and physical signs listed above. Collection of just a single fish may not be representative of the fish population and may result in a wrong diagnosis.
Collection of a sick fish found at the surface or water's edge is best. Collecting fish with a seine or hook and line is not recommended because most fish collected this way will be healthy. Do not collect dead fish; they are useless for the diagnosis of a disease problem.
How can an infectious disease be diagnosed?
If you suspect an infectious-disease outbreak because of clinical signs, send fish samples to a disease specialist for analysis. Successful diagnosis largely depends upon the quality of the specimens collected and how they were handled.
The following are the recommended packaging and shipping methods for sick or diseased fish to the disease diagnostic laboratory. They are listed in order of preference, from excellent to fair.
Live fish
Place the smallest size of affected fish in a strong plastic bag with a minimum amount of water (not more than one-third full). Fill the bag with pure oxygen, tie it securely, place it in another plastic bag and seal it. Place the double bag in a strong cardboard, plastic or Styrofoam® box with insulation. During summer months, pour crushed ice into a separate plastic bag and place it in the box next to the fish bag.
Mark the package "Scientific Specimens — Perishable" and ship by bus or express mail. If shipped by bus, choose a schedule that allows delivery during working hours. Add "Please Call Upon Arrival" and the laboratory's phone number to the package's label.
Iced specimens
Place fish collected in the field in separate plastic bags. Add a small amount of water to prevent the fish from drying out. Seal the bag and place it on crushed ice. Ship in a well-insulated container with sufficient ice to keep the specimens cool until arriving at the laboratory. If you use this method, use a water-tight container.
Frozen specimens
This is the least desirable method. When shipping frozen fish, collect them alive, place them in a plastic bag with a small amount of water and freeze. As soon as possible, ship as instructed above or pack the fish in 3 pounds to 5 pounds of dry ice, which will keep specimens frozen for 24 hours to 36 hours if the shipping carton is well-insulated.
Do not ship frozen fish without dry ice around them. Also, do not ship iced fish that may be delayed in getting to the diagnostic laboratory. The fish will have begun decomposing and will be unsuitable for diagnosis. If there is doubt that the frozen or iced fish will arrive in a condition suitable for diagnosis, the fish should be preserved in 10 percent formalin or 70 percent alcohol and shipped in a strong, sealed plastic bag within another container.
Water sample
In a separate container, include a water sample with the fish specimens. Collect two 1-pint water samples at opposite ends of the pond. Check the dissolved oxygen level at the pond bank because it will change during shipment to the laboratory.
Laboratory location
Lincoln University conducts fish disease diagnosis. A Fish Health Laboratory was set up in 2005 within their Small Animal Research Facility. The lab performs fish health services including quarantine, microbiological analysis, sensitivity testing, parasite identification, water quality analysis, fish necropsies and Coulter Counter analysis of fish blood and fluids. For more information on these services and address to use to ship samples contact the Fish Health Laboratory.
If the diagnostic laboratory is within an hour's drive, place the sick fish in a plastic bag with water and oxygen. Seal the bag and place it in an insulated shipping container or cooler with ice. Use the fastest method possible to get the fish samples to the diagnostic laboratory. Samples must be analyzed within 48 hours to obtain accurate results.
What if water quality is normal and there is no indication of infectious disease?
Stressed, sick, dying or dead fish are strong evidence that a toxic substance is present. Fish may have physical signs that are different from those caused by infectious diseases. Some of the clinical signs are listed in Table 1. However, some toxic substances may produce few or no physical signs.
Table 1
Clinical signs of toxic substances.
| Sign | Possible cause agent |
|---|---|
| white film on gills or skin | acids, heavy metals or trinitrophenols |
| sloughing of gill epithelium | copper, zinc, lead, ammonia, detergents or quinoline |
| clogged gills | turbidity or ferric hydroxide |
| bright red gills | cyanide |
| dark gills | phenol, naphthalene, nitrite, hydrogen sulfide or low oxygen |
| hemorrhagic gills | detergents |
| distended opercles | phenol, cresols, ammonia or cyanide |
| blue stomach | molybdenum |
| pectoral fins moved to extreme forward position | organophosphates or carbamates |
| lip papillomas or skin tumors | polynuclear aromatic hydrocarbons |
If infectious disease is present, should I still be concerned about toxic substances?
Yes. Usually, an infectious disease indicates the possibility of another predisposing environmental condition, such as poor water quality or toxic substances. These weaken the fish and render them susceptible to disease. For example, a toxic substance may be introduced at sublethal levels and accumulate in the fish over time through the food chain.
If another predisposing environmental condition cannot be identified, and if water quality is normal, the presence of a toxic substance is still possible. Therefore, you may collect two additional fish samples for toxic-substance analysis.
How do I collect, preserve and package fish samples for toxic-substance analysis?
Collect two samples, one for organic analysis and one for inorganic analysis. Use the techniques described previously.
Each sample should contain three fish to six fish. The total weight of each sample should be at least 2 ounces. Dead fish can be used if you can verify that the fish just died (they still have red gills and somewhat normal amounts of mucus and color).
For the fish to be analyzed for organic contaminants, rinse the fish with clean water and wrap them in aluminum foil, with the dull side of the foil toward the fish. Freeze the fish as quickly as possible.
For the fish to be analyzed for inorganic contaminants, rinse the fish with clean water, place them in a polyethylene bag and freeze. Use caution when collecting samples from a suspected toxic-substance fish kill.
Pack the frozen samples in dry ice in an insulated shipping carton. The samples must remain frozen until received by the toxic-substances laboratory.
If a toxic substance is suspected, do I also need to collect water samples?
Yes. Fish break down or metabolize some organic toxic substances very rapidly. The original compound may not be identifiable from the fish analysis. In these cases, analysis of water samples helps identify the toxic contaminant.
Collect two water samples of 1 quart each. One sample will be analyzed for organic contaminants, and the other sample will be tested for inorganics, such as heavy metals.
Use properly cleaned and prepared containers. You may obtain these from the toxic-substances laboratory or commercially. The containers have been through rigorous cleaning procedures that remove background levels of contaminants.
Collect the water to be analyzed for organic contaminants from the surface of the pond or lake. Precleaned amber glass bottles are usually required. Fill the bottle to overflowing. Cap it with a Teflon®-lined cap. Do not include disturbed sediment, outboard motor oil or similar substances that may hinder the identification of the relevant contaminant.
Place the water sample to be analyzed for inorganics in a plastic bottle. Collect surface water and fill to the bottle's shoulder area. Add 10 milliliters of nitric acid to preserve the water. Ampules that contain premeasured amounts of acid are available commercially. Cap the bottle and shake it.
Pack both samples in ice in a Styrofoam®-lined shipping carton (hard-cased coolers may be used). The samples must remain cold but not frozen because freezing can break the glass bottles. Send the water samples along with the samples of frozen fish.
If legal action is a possibility, check with the toxic-substances laboratory for instructions on applying chain-of-custody security seals and filling out required custody documentation. Ship the cartons or coolers by the fastest method — overnight mail or one-day delivery service.
What information should be included with the samples?
With each fish sample sent to the fish-disease diagnostic laboratory or to the toxic-substances laboratory, submit the following information:
- Name, address and phone number of the submitter.
- Date of collection.
- Designation of pond or tank from which fish were removed. Labels of samples must include the identity of tanks or ponds.
- Dimensions of pond or tank, including depth.
- Species, number and average size of fish.
- Date of last fish stocking, including number, species and size.
- Amount of food fed per day. Indicate if fish are still eating or when they stopped eating.
- Date first mortalities observed.
- Approximate number of dead fish per day since mortalities first observed.
- Identify most recent chemical treatment, including date and amount.
- Determine clarity of water by Secchi disk depth.
- Provide water quality data of ponds or tanks.
Label water samples for the toxic substances laboratory with the following information:
- Name, address and telephone number of submitter.
- Date of collection.
- Designation of pond or tank from which water was collected.
- Method of preservation (ice, acid, etc.).
What steps can be taken to control losses while awaiting results?
The length of time for laboratories to complete analyses varies. Samples sent to the fish-disease diagnostic laboratory should have necropsy and parasitology results within 24 hours after receipt of the fish. Usually, microbiology (bacterial isolation and sensitivity) results are complete after 48 hours to 96 hours, but virology (identification of virus) and histopathology (microscopic examination of specially prepared tissues) may take one week to two weeks for completion.
Typically, delivery of test results from the toxic-substances laboratory is within 90 days. You may send samples in the "expedited-" or "rush-"analysis category, which usually provides results within 30 working days at an additional cost.
It is best to improve water quality while waiting for diagnostic and/or toxic substances results. Often, increased aeration and flushing with fresh water can reduce the severity of many problems.
Conclusions
Fish mortalities occur in natural populations and under aquaculture conditions. They are caused by a variety of factors. In aquacultural facilities, good management and nutritional practices can help prevent fish kills. Usually, when fish kills occur on a fish farm or in a private pond or lake, it is an emergency situation.
At the first sign of a problem, poor water quality should be eliminated as a potential cause. If infectious disease is present, look for an adverse condition that could have weakened the fish and triggered the disease, such as crowding or poor food quality. If no adverse condition is identified, a toxic substance may be suspected. In the case of good water quality and no infectious disease, the presence of a toxic substance is strongly indicated.
To optimize the response to a fish mortality problem, producers must be prepared to check water quality parameters and obtain a fish and a water sample, transporting them as quickly as possible to a diagnostic laboratory.
However, collecting fish and water for toxic-substance analysis involves special pre-cleaned containers and specific preservation and shipping requirements. Advanced preparation is crucial to successful sample collection and analysis to solve the problem.
References
- A Guide to Integrated Fish Health Management in the Great Lakes Basin. 1983. Fred P. Meyer, James W. Warren and Timothy G. Carey, Great Lakes Fishery Commission.
- Fish Hatchery Management. 1982. Piper et. al., U.S. Department of Interior, Fish and Wildlife Service, 517 pp.
- The Third Report to the Fish Farmers. 1984. Harry K. Dupree and Jay V. Huner, U.S. Department of Interior, Fish and Wildlife Service, 270 pp.
- Field Manual for the Investigation of Fish Kills. U.S. Department of Interior, Fish and Wildlife Service, Resource Publication 177.